SELLING OFFER
Seek co-innovation with global enterprises and governmental institutions
NRDO (No Research Development Only) based technology incubation service for Medtech and Digital Healthcare startups
374 2023. 6. 5.
Summary
The company was founded in 2020 by engineers from Samsung Electronics who worked together to support the technology development of deep tech startups.
The company are doing a NRDO (no research development only) service to create customized Business Service that solves common difficulties from planning to development, manufacturing, and sales.
Files(Download)
Advantages and Innovations
By doing this service, the company will be able to have below advantages:
1) Solving engineering problems in hardware startups.
2) Providing customized Business Service that solves the difficulties of startups from planning to development, mass production, manufacturing, certification, and sales.
3) The company has a team of experienced engineers (AI, Software, Mechatronics and Semiconductor design to identify and advance potentially transformative medicines) from Samsung Electronics.
4) Technology Incubating (development and certification of commercialization of medical devices using digital technology such as especially “optics”) for medical device and healthcare startups.
5) Incubating the next-generation “big medtech and digital healthcare” company, based on well experienced and essential technology in terms of electronic system, mechatronics, optic, data base and networking to enhance the technical capability of Medtech and Digital Healthcare Startups having very innovative business model through specific science and research study.
The company are developing medical technology 1) bio information sensing and analysing platform covering wearing device and data base networking 2) optical diagnose medical device for dentistry, ophthalmology, and dermatology) and product based on process, standards and regulation for GMP, FDA and MDR.
Stage of Development
Description
The company offers a service for technology Incubating (development and certification of commercialization of medical devices using digital technology such as optics) for medtech and digital healthcare startups.
The target clients are:
1) SMEs or startups that have deep technology and deep science, such as medical devices, but do not have practical experience from actual planning to development, mass production, manufacturing, certification, and sales to realize them in detail.
2) Overseas companies that need research, or are seeking international joint research.
3) Foreign investors who want to discover and invest in these medical device deep-tech companies.
Our client`s technology is as follows:
- Electronic System
- System analysis (understanding the product)
- Establishment of optimal design plan
- Required schedule, resource analysis
- Design for Manufacturing
- Establishment of mass production specifications
- Schematic/PCB design
- SW, FW design
- Product Layout Review
- V&V (SET performance verification)
- Optical System
- Optical Specification Review
- Optical structure design (optical path analysis)
- Core parts (lens, filter) design
- Adjustment, jig design
- Mass production process design
- V&V (Performance Verification)
- DB & Networking
- IoT sensor review
- Tracking Specifications
- DB, server construction
- UI (Dashboard Design)
- APP, WEB design
- Networking Protocol (FHIR)
- Security
- Mechatronics
- Review of instrument specifications
- Mechanical structure design (optical path analysis)
- Core parts, mold design
- Facade/frame design
- Mass production process design
- V&V (Performance Verification)
- Portfolio (Product & Technology)
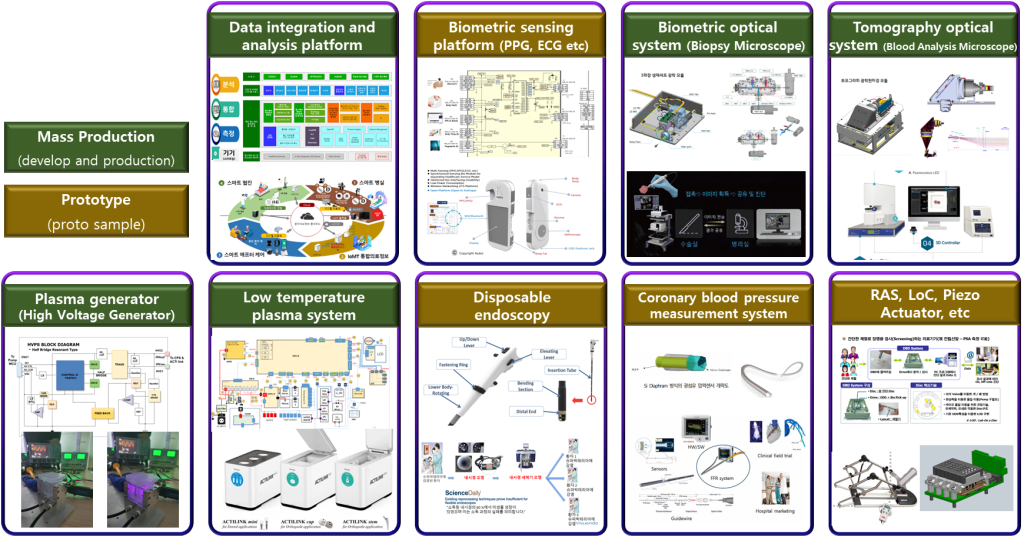
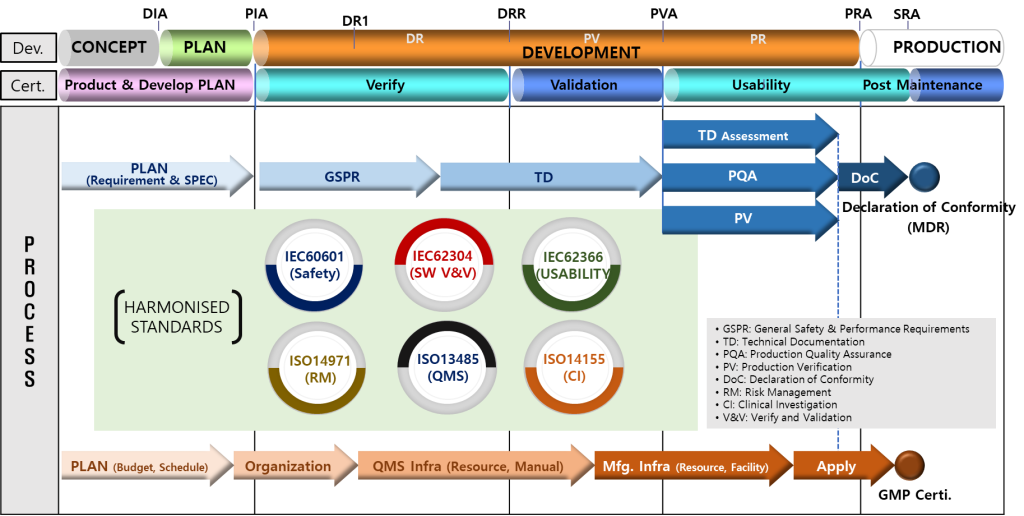
- MEDDEV Development Process
- Performance
1) Carried out technology incubation to enable IPO of 2 out of 3 of the company's flagship portfolios (Tomocube, Plasmapp)
2) Performed 5 mass production R&D projects and 4 prototype projects
3) Proceed with technology development according to the international standard quality process for easy and smooth medical device approval of MDR, FDA etc.
This technology service contracts to provide technology, investment business contracts to discover and invest in start-ups to create value, and international joint research, technology development specialized in medical devices, certification or investment, joint research, etc.
Technology Keywords
Market Application Keywords
Sector Group
Type and Size of Client
Type and Role of Partner Sought
- Type of partner sought: Companies, public institutions, private institutions which deals with Medtech;
- Specific area of activity of the partner: Medtech startups having difficulties due to a lack of experiences such as practical experience from actual planning to development, mass production, manufacturing, certification, and sales to realize them in detail, especially commercialization, mass production, and certification of medical devices.
Type of Partnership Considered
Financial agreement
Research cooperation agreement
Company
Internal Reference
Category
Express your interest in this opportunity.