SELLING OFFER
Seek co-innovation with global enterprises and governmental institutions
Wearable device for chronic migraine treatment
188 2023. 5. 24.
Summary
The company conducts both animal studies and clinical trials in partnership with renowned university-hospitals in Korea. A wearable prophylactic and acute treatment device for chronic migraine is manufactured in a MDSAP approved manufacturing facility in Korea. The company seeks distributors for the EU region.
Advantages and Innovations
Economic:
- Compared to the existing treatment options, it’s far more affordable.
No adverse effects:
- There are no known permanent or serious adverse effects reported by wearing the treatment device.
Effective:
- The treatment device can have the acute symptom relieved by wearing the device for 10 minutes and have the preventative effect by wearing the device for two weeks.
Stage of Development
Description
The device has two protocols of treatment: preventive and acute protocols, like the typical method of treatment for chronic migraine. By wearing the device for 30 minutes, the device transcutaneous delivers electric current on trigeminal nerve fibers and modulates the autonomic nervous system. All typical types of migraine patients can use the device. It shortens migraine duration, reduces the number of migraine attack, and weakens attack intensity.
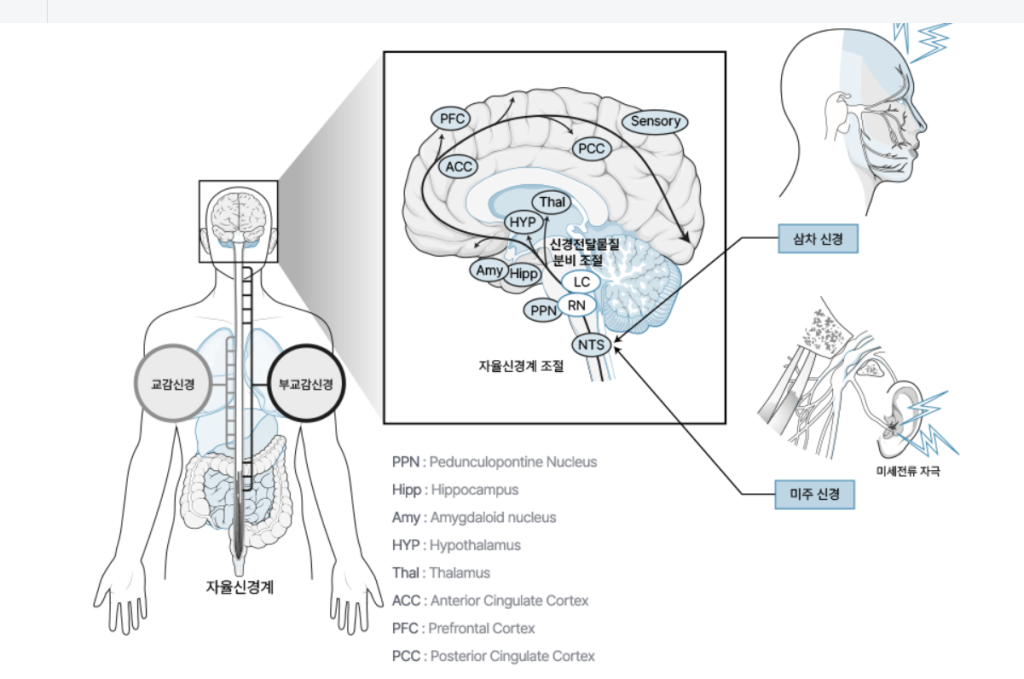
It is a medical device in Korea, the US, Europe, and Canada. As approved in the EU region, distribution and sales are needed.
Technology Keywords
Market Application Keywords
Sector Group
Type and Size of Client
Type and Role of Partner Sought
- Type of partner sought: Medical Device Distribution, Retails Companies
- Specific area of activity of the partner: Medical Device
Type of Partnership Considered
Company
Internal Reference
Category
Express your interest in this opportunity.