SELLING OFFER
Seek co-innovation with global enterprises and governmental institutions
ETC and OTC treatments for skin diseases
182 2023. 8. 14.
Summary
The company is a Korean-born company mainly responsible for R&D (medicine, biomedical engineering, and chemistry), and has a branch office in the United States.
As a product developed in Korea, it can be applied for both prescription (ETC) and non-prescription (OTC) purposes by solving the side effects of retinoid-based acne and treatments and minimizing toxicity.
We are currently developing production and quality control (CMC) data, and we are first seeking funding for clinical research for OTC monograph approval by the US FDA or EMA.
Advantages and Innovations
- This product is available both prescription and Over-The-Counter.
- Can be used to treat complex symptoms such as acne and melasma.
- Since the main ingredient dissolves well in water, the ingredient remaining on the skin surface is less irritating to the skin, so there are fewer side effects or toxicity.
- Compared to existing tretinoin products on the market, more than 3 times the effect has been confirmed.
- This product has 4 patents registered at the Korean Intellectual Property Office related to synthetic technology and efficacy, and is internationally protected by PCT.
Stage of Development
Description
The company was founded in 2013. Currently located in the United States and Korea, the Korean office is mainly responsible for R&D, and the US office is responsible for technology possession, FDA information collection, regulation and certification. It's like a Q&A.
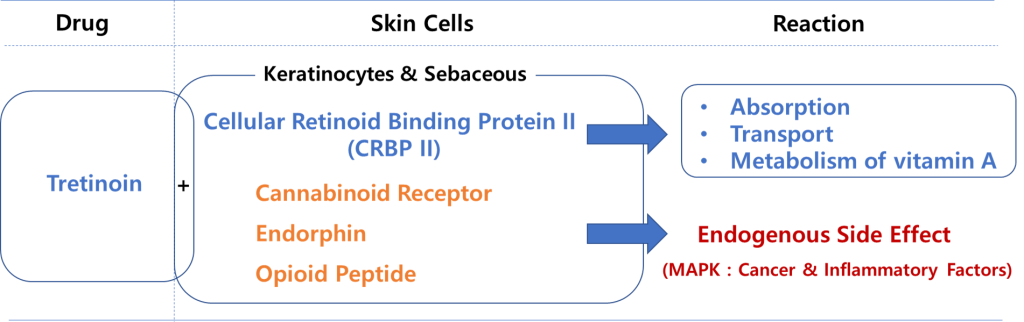
Common acne treatments use “tretinoin” in their ingredients. Tretinoin is a topical retinoid, a class of medication known to help treat acne and inflammation by increasing skin cell turnover.
Tretinoin has a whitening effect that brightens the skin, changes old skin to new skin, and protects skin damaged by the sun.
However, tretinoin has very serious inflammatory side effects in certain skin populations. Also, since it is not classified as an over-the-counter drug, Asian consumers purchase it without a prescription and mix it with cosmetics for whitening purposes.
Most retinoid acne treatments are ETC (prescription drug) drugs, but in 2016, Adapalene was first approved as an OTC drug, but it does not have skin penetration.
The company's developments are ETC and over-the-counter (OTC) medications designed for use in the treatment of all types of acne and melasma.
This product has high penetration and residual power, so the efficacy is good, and the side effects and skin irritation index are as low as OTC level.
Based on the cell experiment of this product, the formulation was developed at the National Graduate School of Pharmacy in Korea. In an experiment comparing the stability and absorption rate of the most common and well-known tretinoin product with animal skin, this product was about 30% higher than the tretinoin product.
The endogenous and exogenous mechanisms of action of this product have been proven, and safety, skin permeability, topical toxicity, and efficacy evaluation tests have been completed in accordance with regulatory standards.
Technology Keywords
Market Application Keywords
Sector Group
Type and Size of Client
Type and Role of Partner Sought
Clinical trial supervisor for OTC Monograph approval in Europe
Type of Partnership Considered
License Agreement
Company
Internal Reference
Category
Files(Download)
If interested, please press this button.
We will answer you soon.